
Formaldehyde Monitoring
Formaldehyde (CH2O), also known as methanal, is a colorless, toxic gas with a distinct, strong pickle-like pungent odor. It is released from emissions of almost every volatile organic hydrocarbon, during the decay of plant material in the soil and is found in tobacco smoke. Prolonged exposure to CH2O can cause watery eyes, burning sensations in the eyes and throat, nausea, and difficulty in breathing, and may lead to cancer, making the use of formaldehyde monitors a viable solution to provide adequate warning of hazardous exposure. This article covers information on formaldehyde, its sources in the ambient air, permissible levels, health and environmental impact, possible corrective measures, the need for formaldehyde monitors as well as different methods of CH3SH monitoring.
What is CH2O?
Formaldehyde, also known as methanal, is a naturally occurring organic compound with the formula CH2O (or H−CHO). It is a colorless chemical with a strong pickle-like pungent odor that is commonly useful in many manufacturing processes. Its pure gaseous form polymerizes spontaneously into paraformaldehyde, hence it is usually stored in its liquid form, called formalin. Also, it is one of the most common volatile organic compounds (VOC) found in the atmosphere
The main chemical and physical properties of CH2O are as follows :
- Molecular mass = 30.03 g/mol
- Relative vapor density = 1.03-1.07 (air = 1)
- Melting point = −92 °C and boiling point = −19.1 °C.
- Soluble in water (around 400 g/l at 20 °C), ethanol, and chloroform and miscible with acetone, benzene, and diethyl ether.
Formaldehyde dissolves easily in water (known as formalin) but does not last long there, either. Formalin is commonly useful as an industrial disinfectant and as a preservative in funeral homes and medical labs. When an item gives off formaldehyde, it is released into the air through a process called off-gassing. Formaldehyde gas quickly breaks n in the air - generally within hours.
Formaldehyde in the Atmosphere
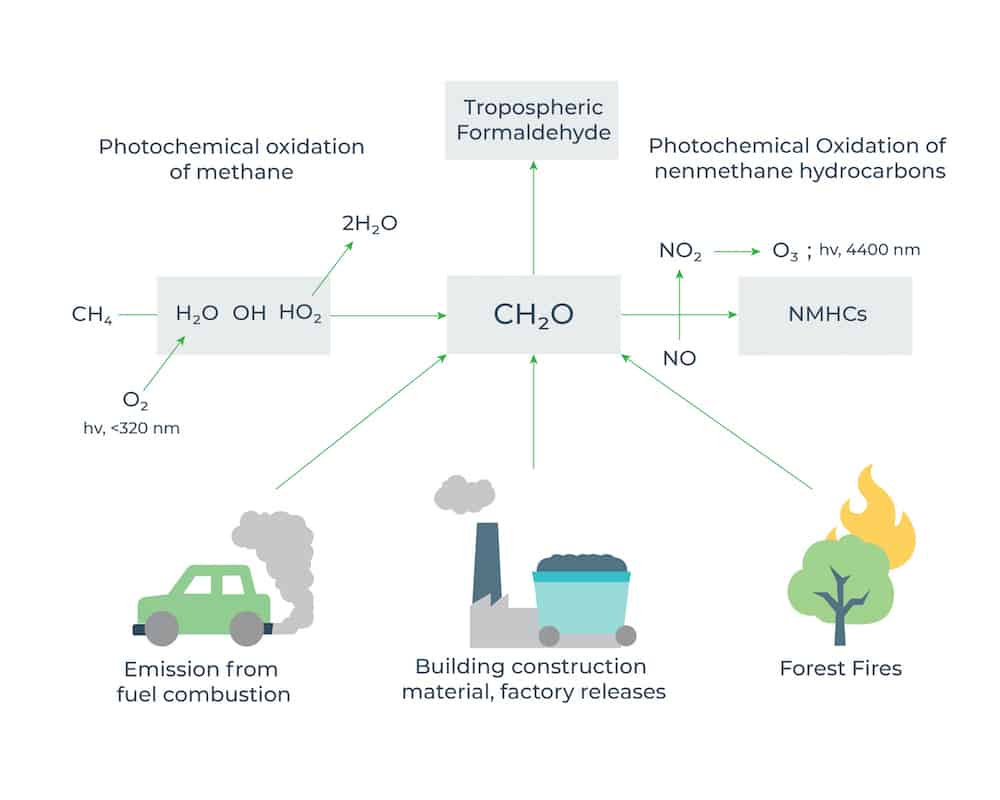
Formaldehyde is the most common aldehyde in the environment. The natural background concentration is < 1 µg/m3 with a mean of about 0.5 µg/m3. [Ref- Formaldehyde. In: Wood dust and formaldehyde. Lyon, International Agency for Research on Cancer, 1995, pp. 217-362.]
Being a ubiquitous trace chemical in the ambient atmosphere, formaldehyde (CH2O) plays an important role in atmospheric photochemistry because it reacts quickly in the atmosphere and its photolysis provides new hydroxyl (OH) and hydroperoxy radicals (HO2). CH2O can also influence secondary organic aerosol (SOA) by providing radicals that increase gas-phase oxidation of hydrocarbons and increase surface-active organic material. When produced in the atmosphere by the action of sunlight and oxygen on atmospheric methane and other hydrocarbons, it becomes part of smog. Formaldehyde does not accumulate in the environment, because it is broken down within a few hours by sunlight or by bacteria present in soil or water.
Sources of Formaldehyde
Natural Source
Formaldehyde occurs naturally in the environment. It is produced in the atmosphere from emissions of almost every volatile organic hydrocarbon. It is also produced during the decay of plant material in the soil and during normal chemical processes in most living organisms. Formaldehyde is a combustion product found in tobacco smoke. Humans and most other living organisms make small amounts as part of normal metabolic processes.
Anthropogenic Sources
Anthropogenic sources include direct ones such as on-site industrial emissions and fuel combustion from traffic. Other combustion processes (power plants, incineration, etc.) also represent sources of formaldehyde emissions in the atmosphere. However, formaldehyde is also extensively produced industrially worldwide for use in the manufacture of resins, as a disinfectant and fixative, or as a preservative in consumer products. The most significant sources of formaldehyde are likely to be pressed wood products made using adhesives that contain urea-formaldehyde (UF) resins.
Formaldehyde and other chemicals that release formaldehyde are sometimes used in low concentrations in cosmetics and other personal care products like lotions, shampoo, conditioner, shower gel, and some fingernail polishes. Formaldehyde is also a component of tobacco smoke and both people who smoke and those breathing secondhand smoke are exposed to higher levels of formaldehyde. A study published in 2009 by researchers of Masonic Cancer Center, University of Minnesota found much higher levels of formaldehyde bound to DNA in the white blood cells of people who smoke compared to those who don’t smoke.
Permissible levels of Formaldehyde
Below are the permissible exposure limits of Cl2 in terms of continuous occupational exposure:
Permissible Exposure Limit (PEL) given by OSHA (Occupational Safety and Health Administration) defines the maximum concentration of CH2O to which an unprotected worker may be exposed to. PEL may reference an eight-hour time-weighted average (TWA), a 15-minute short-term exposure limit (STEL), or an instantaneous ceiling limit (CL) concentration that cannot be exceeded for any period of time. Similarly, Recommended Exposure Limit (REL) is the occupational exposure limit recommended by NIOSH (National Institute for Occupational Safety and Health) and the TLVs (i.e. threshold limit values) are the exposure guidelines given by ACGIH (American Conference of Governmental Industrial Hygienists)
Health & Environmental Impacts of Formaldehyde
Health impact
Health effects include eye, nose, and throat irritation; wheezing and coughing; fatigue; skin rash; severe allergic reactions. Formaldehyde can cause watery eyes, burning sensations in the eyes and throat, nausea, and difficulty in breathing in some humans exposed at elevated levels (> 0.1 ppm). High concentrations may trigger attacks in people with asthma. There is evidence that some people can develop a sensitivity to formaldehyde. It has also been shown to cause cancer in animals and may cause cancer in humans
Environmental Impact
Typical release of low concentrations of formaldehyde gas is unlikely to impact the plant and wildlife in the vicinity as it quickly reacts to form other products in the atmosphere. It usually breaks down quickly to create formic acid and carbon monoxide. As a volatile organic compound, it is involved in the production of ground-level ozone, which can damage crops and materials as well as result in the production of other harmful air pollutants.
Possible corrective measures
The primary action is formaldehyde monitoring i.e. to measure how much CH2O concentrations you are exposed to. In addition to this, you can take the following corrective measures:
- Ventilation - Ventilation is the most widely used control method for reducing the concentration of airborne formaldehyde. There are two primary types of ventilation,
- Local exhaust ventilation: It is designed to capture airborne formaldehyde as near to the point of generation as possible.
- General dilution ventilation: It involves the continuous introduction of fresh air into the exposure area/source to mix with the contaminated air and lower the concentration of formaldehyde.
- Substitution - One of the most effective methods of controlling exposure to formaldehyde is to substitute a safer, less toxic material where possible.
- Isolation - People exposed may be isolated from direct contact with the source environment by the use of automated equipment operated by personnel observing from a closed control booth or room.
- Avoid smoking - Formaldehyde is one of the main air pollutants generated by tobacco smoking.
Measurement methods of Formaldehyde monitoring
Different working principles for methyl mercaptan monitoring in the ambient environment are chemiluminescence, semiconductor, and electrochemistry.
Proton transfer reaction mass spectrometry (PTR-MS)
This method of formaldehyde monitoring uses proton-transfer reactions with H3O+ (hydronium) ions to ionize atmospheric formaldehyde molecules and mass-spectrometric detection of the product ions to know the concentration of CH2O. The formaldehyde monitor based on this principle consists of an external ion source, a drift tube, and a mass analysis detection system.
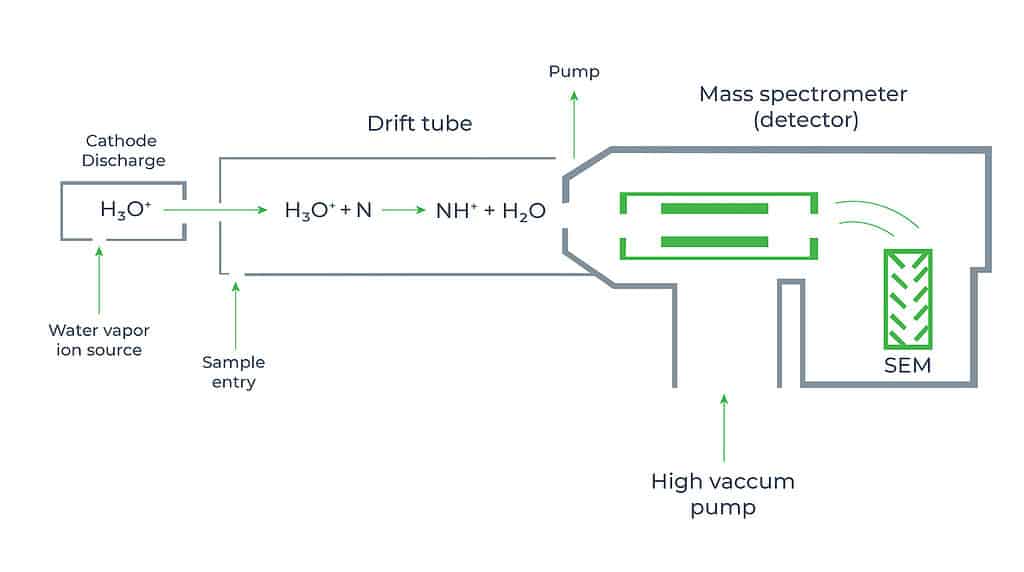
The proton-transfer chemistry takes place in a drift-tube reactor, which enhances the ion kinetic energy and effectively limits cluster ion formation with the abundant water molecules in ambient air. This simplifies both the proton-transfer chemistry and the interpretation of the mass spectra. The exothermicity of the proton-transfer reactions is low enough that the extent of product ion fragmentation is limited and product ion masses can be used as a unique identifier to detect the formaldehyde concentration. However, the slightly higher proton affinity of formaldehyde than that of water leads to low and strongly humidity-dependent sensitivity of formaldehyde in such formaldehyde monitors.
Semiconductor
When a metal oxide semiconductor-based formaldehyde monitor is exposed to the air sample, the CH2O molecules react on the metal oxide surface of the sensor and dissociate into charged ions which alter the resistance of the film. This interaction is measured as a signal and is converted to the gas concentration. However, the energy consumption of such formaldehyde monitors is higher compared to others.
Electrochemical
CH2O monitors working on the electrochemical principle are operated based on the diffusion of formaldehyde gas into the sensor which results in the production of electrical signals proportional to the CH2O concentration. It allows accurate measurement of even low concentrations of CH2O, which is essential in formaldehyde monitoring in the ambient air.
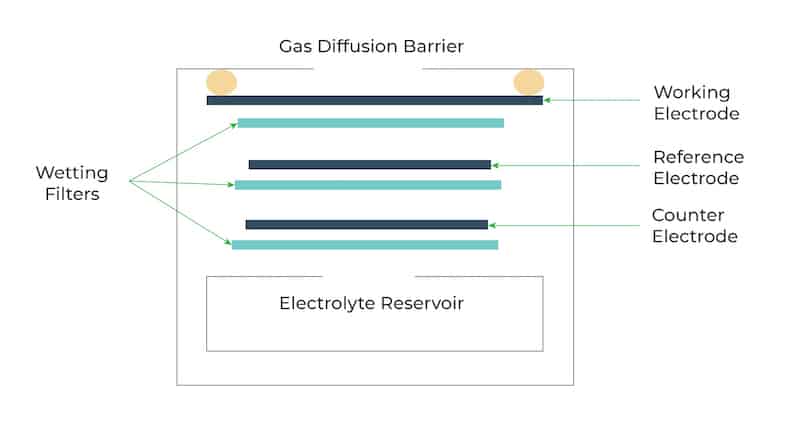
Among all the above principles of formaldehyde monitoring, CH2O monitors based on electrochemistry are typically found to be preferred for ambient air monitoring as they yield more accurate CH2O concentrations and are inexpensive in comparison with the others.
Oizom’s working principle for Formaldehyde monitoring
Oizom’s ODOSENSE is a real-time odor emission tracking solution. It continuously detects, measures, and monitors the odourful gaseous contaminants including hydrogen sulfide, ammonia, sulfur dioxide, methyl mercaptan, TVOC, formaldehyde, methane, and weather parameters like temperature, humidity, wind speed, and wind direction. The sensor that measures CH2O works on the principle of electrochemical sensing. With the help of meteorological data, Odosense can trace the odourant dispersion plume incited by conditions like wind speed and wind direction. Furthermore, it is a proactive approach to measuring real-time odor emissions. This makes it an ideal choice for landfill sites, wastewater treatment facilities, fertilizers, paper-pulp industries, soil-treatment sites, etc.
5 Reasons Why Formaldehyde monitoring is important
- Formaldehyde is a colorless gas with a strong pickle-like pungent odor. It easily reacts in the atmosphere to form other compounds or breaks down in soil and water within days of emission.
- People may be exposed to formaldehyde by breathing contaminated air from sources such as pressed-wood products, tobacco smoke, and automobile tailpipe emissions
- Exposure to formaldehyde causes irritation to the eyes, and skin as well as respiratory tract, wheezing, and coughing; fatigue; skin rash; severe allergic reactions, nausea, and difficulty in breathing which can lead to cancer.
- Prolonged exposure to CH2O can quickly deaden a person’s sense of smell, making the odor of methyl mercaptan an unreliable indicator of its presence. Hence, other means such as the use of methyl mercaptan monitors are a viable solution to provide adequate warning of hazardous exposure.
- It is one of the most common VOCs present in ambient air. Real-time monitoring of CH2O levels helps in determining their source as well as formulating an action plan to control methyl mercaptan emissions.
Link nội dung: https://blog24hvn.com/ch2o-a37387.html